Slide
Built on 15 years of experience.
Our Clinical Discovery Platform (CDP) is a rock-solid, technologically superior, and fully compliant clinical trial software – built on 15 years of experience working with the most advanced development tools and processes.
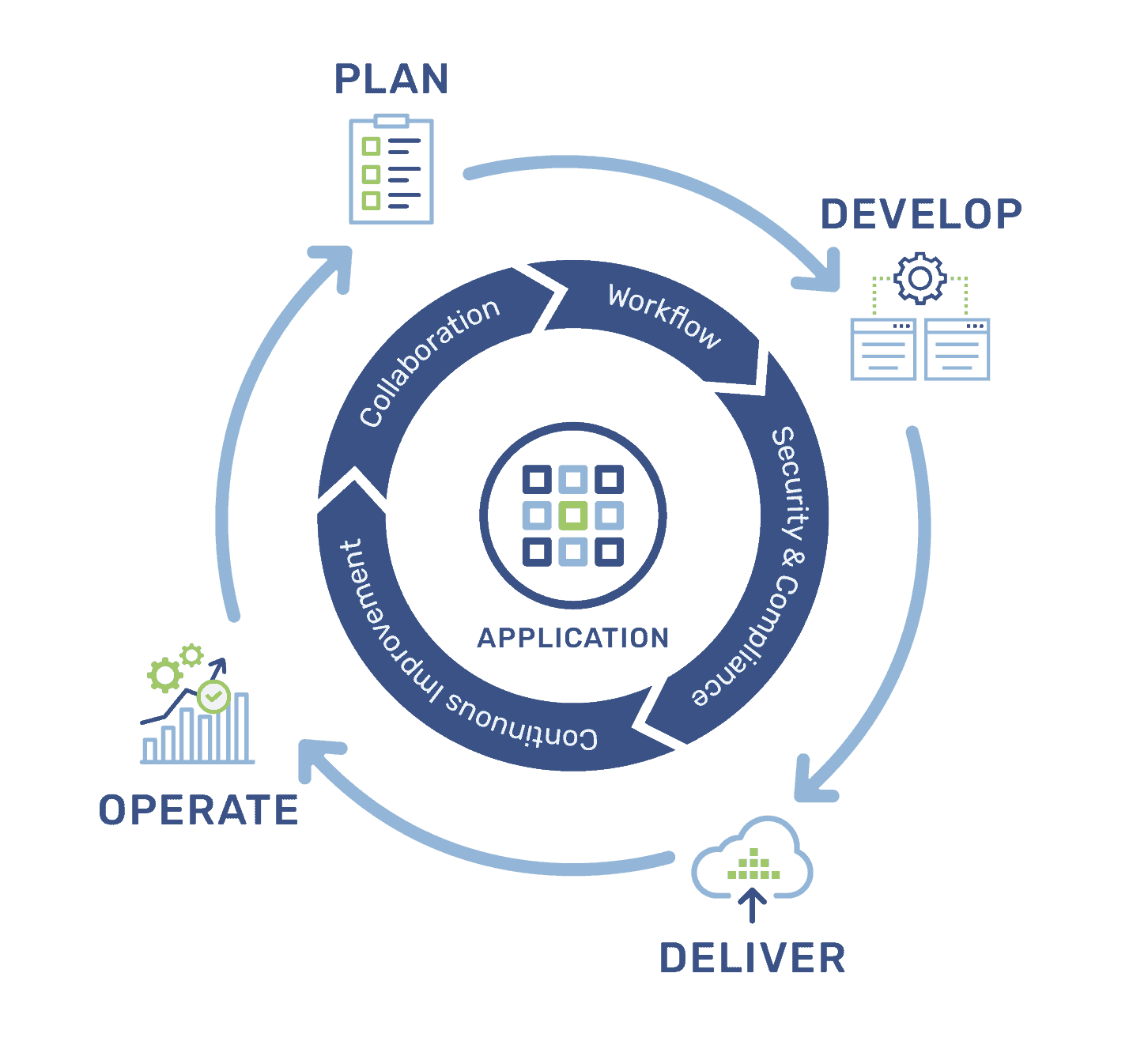
We use end-to-end solutions on Azure to implement DevOps practices throughout application planning, development, delivery, and operations. Applying the right combination of DevOps technologies, culture, and processes to enable continuous software delivery and better solutions for our customers.
Leveraging the full suite of Azure DevOps tools, we empower our team to manage their work with agility and full visibility across products and projects. They define, track, and layout work with Kanban boards, backlogs, custom dashboards, and reporting capabilities using Azure Boards. Our employees keep development efforts transparent and on schedule with GitHub.
Continuous Development/Continuous Integration (CD/CI) from code to cloud, we automate each part of the DevOps process. We have been using Agile software development methods for nearly a decade, with planning, tracking, and reporting for shorter release cycles and full visibility into our software development process.
More From Simplified